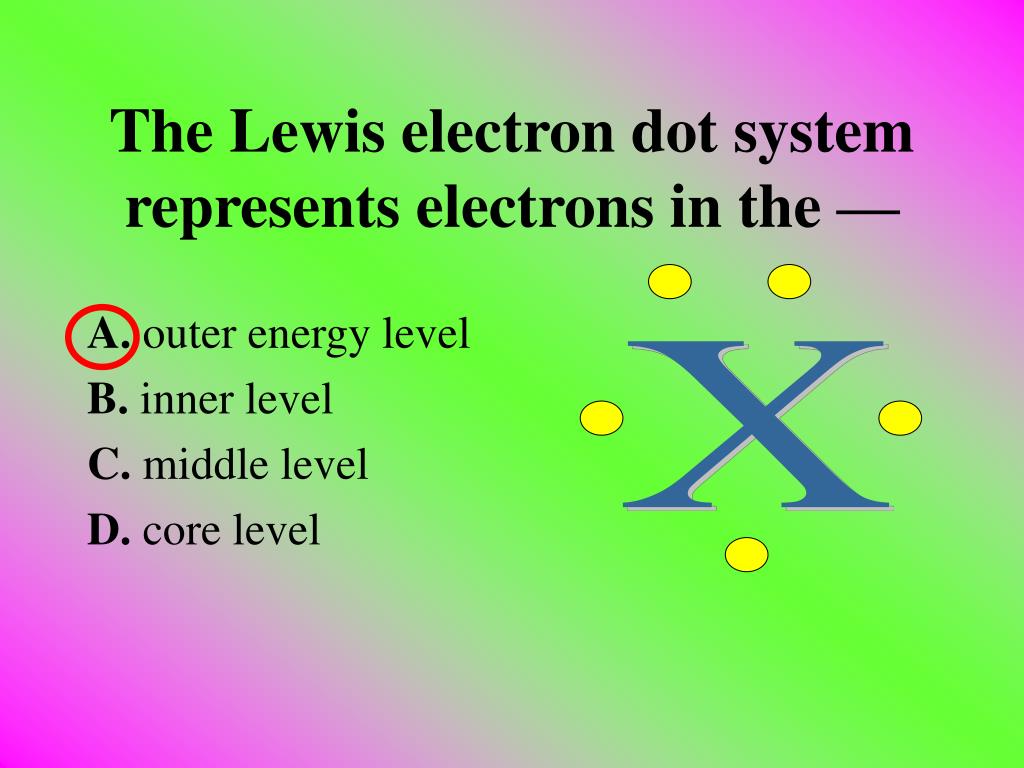
The Lewis Electron Dot System Represents Electrons In The
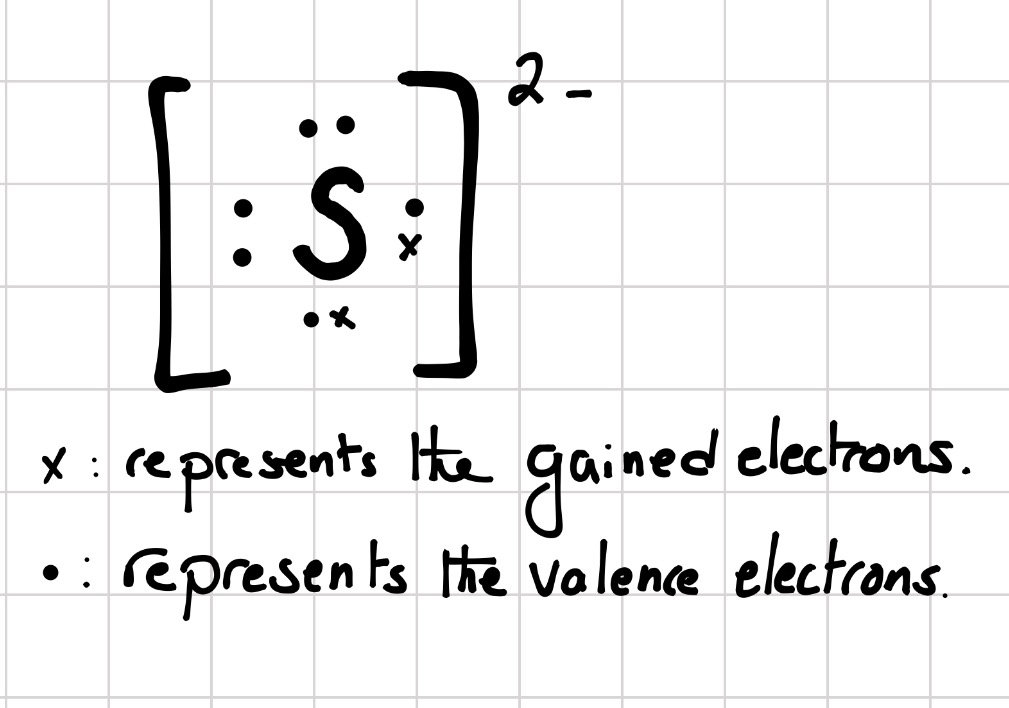
The lewis electron dot system represents electrons in the. Sometimes it is more convenient to represent the element s by its Lew is electron dot symbol. The Lewis electron dot system represents electrons in the. Your response must include th the numerical value and the sign of the charge.
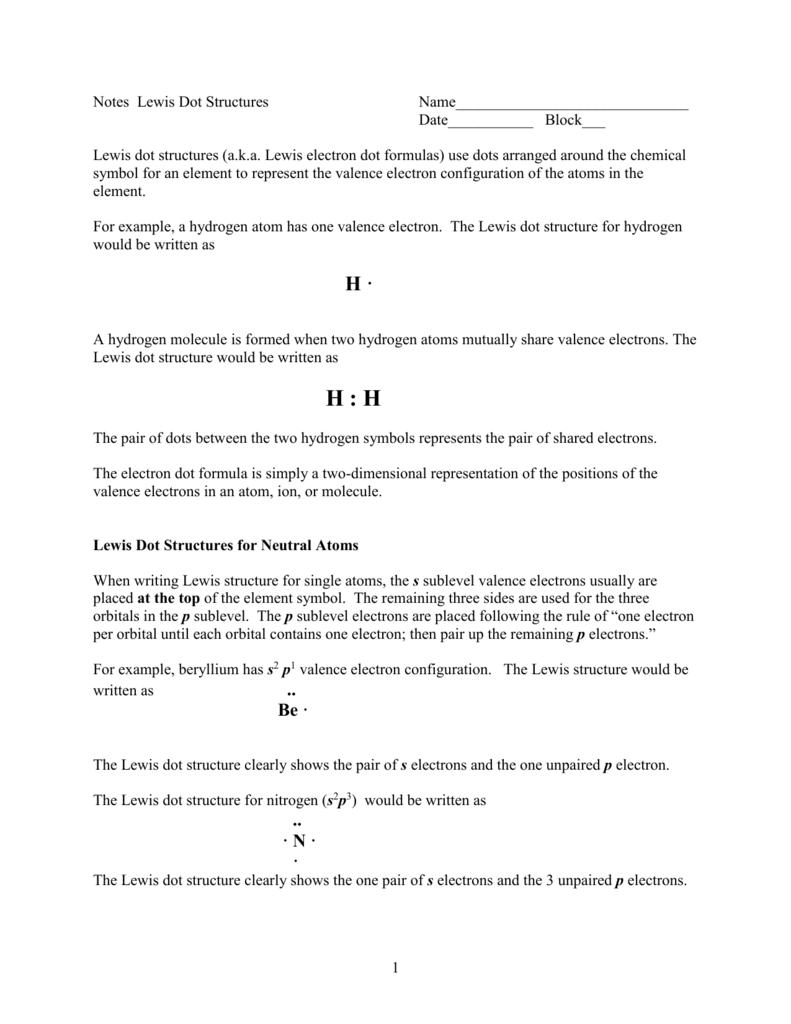
We normally present these Dot Structures as part of an Atomic or Molecular Chemical Structure or to show how different atoms connect to others Chemical Bonding. All the electrons in the outermost sub-level in an atom. It would definitely be helpful if a few rules were followed.
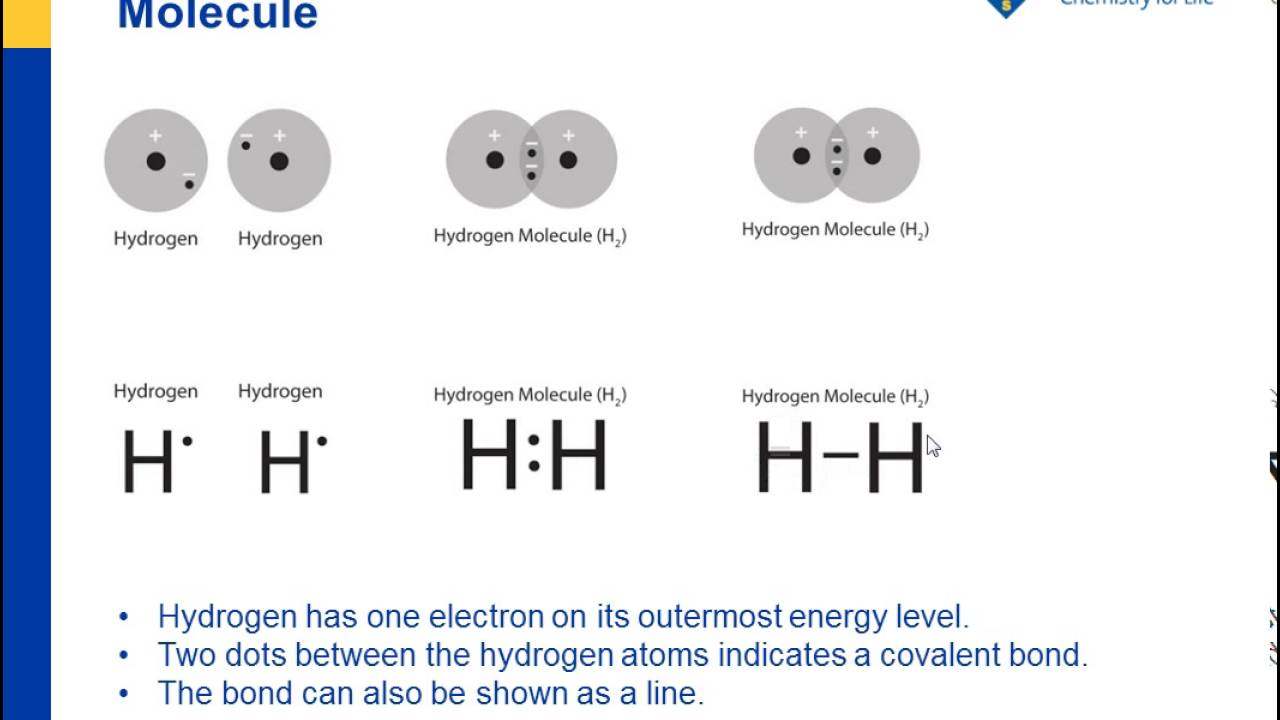
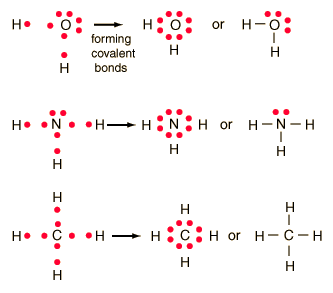
Estimate the observed angle. Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired. Single bonds are represented by a pair of dots or one line between atoms.
Inner-shell electrons cannot be proven to exist and thus should not be included in. All the valence electrons in the. To facilitate our understanding of how valence electrons interact a simple way of representing those valence electrons would be useful.
Each dot represents one electron. When potassium salts are heated in a flame a purple color is observed. Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom.
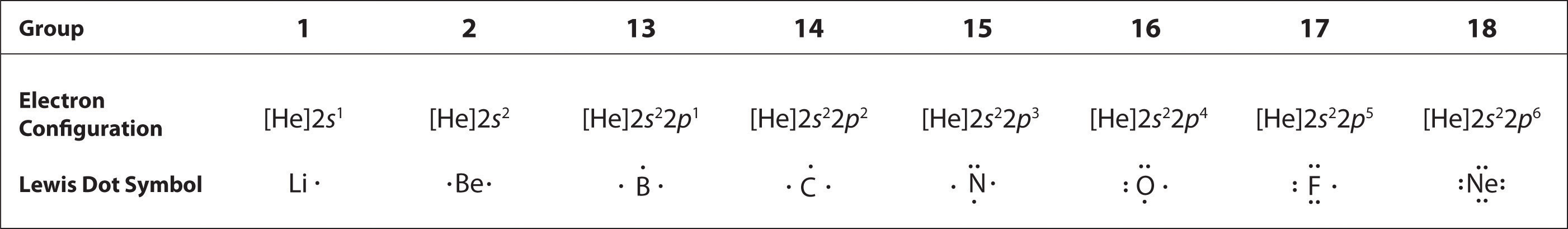
The valence electrons - the ones in the farthest energy level from the nucleus that contains electrons in an atom. Sodium atoms potassium atoms and cesium atoms have the same 1 atomic radius 2 first ionization energy. The Lewis electron dot system represents electrons in the energy level.
Lewis Dot Structure. In Lewis dot structures each dot represents an electron.
To facilitate our understanding of how valence electrons interact a simple way of representing those valence electrons would be useful.
Atoms and Atomic Structure. Inner-shell electrons cannot be proven to exist and thus should not be included in. Ii In the molecule angle y is not 900. Determine the charge of an IJut nucleus. A Lewis electron dot symbol shows. All the electrons in the outermost sub-level in an atom. The tendency to form species that have eight electrons in the valence shell is called the octet rule. The valence electrons - the ones in the farthest energy level from the nucleus that contains electrons in an atom. Sodium atoms potassium atoms and cesium atoms have the same 1 atomic radius 2 first ionization energy.
The Lew is Electron-Dot Symbol s of Element s. The Lewis Electron Dot System Represents Electrons In The What. This rule applies well up to period 4 when it takes 18 electrons to fill the outer orbitals. When potassium salts are heated in a flame a purple color is observed. The octet rule states that atoms with eight electrons in their outer shells are stable. Estimate the observed angle. Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures LEDS are diagrams that show the bonding between atoms of a molecule as well as the lone pairs of electrons that may exist in the molecule.










:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)





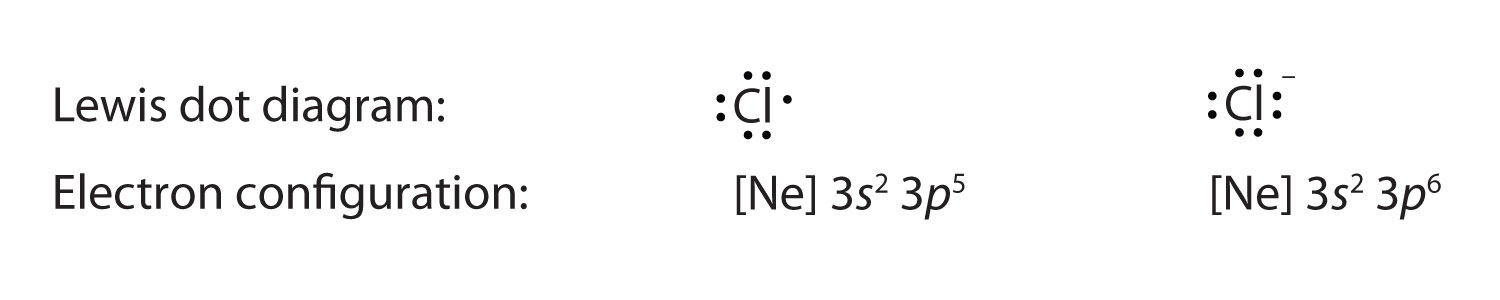







/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)







Post a Comment for "The Lewis Electron Dot System Represents Electrons In The"